Solution:
Bromine water test –
Bromine water is used to differentiate between unsaturated compounds (like alkenes and alkynes) and saturated compounds. For this purpose, bromine is used in the form of bromine water. A solution of bromine in water is called bromine water. Bromine water has a red-brown colour due to the presence of bromine in it. When bromine water is added to an unsaturated compound, then bromine gets added to the unsaturated compound and the red-brown colour of bromine water is decolourised. So, if an organic compound decolorizes bromine water, then it will be an unsaturated hydrocarbon (containing a double bond or a triple bond), but saturated hydrocarbon (alkanes) does not decolourize bromine water.
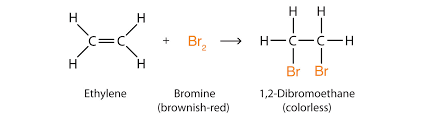
The bromine water test is performed to differentiate between the unsaturated compounds (like alkenes and alkynes) and the saturated compounds. Bromine water is added to an unsaturated hydrocarbon red-brown colour of bromine solution is discharged. So if there is dis-colouration then the compound will be an unsaturated Hydrocarbon.
