THE MODERN PERIODIC TABLE


In 1913, Henry Moseley showed that the atomic number (symbolised as Z) of an element is a more fundamental property than its atomic mass.
Accordingly, Mendeléev’s Periodic Law was modified and atomic number was adopted as the basis of Modern Periodic Table and the Modern Periodic Law can be stated as follows:
‘Properties of elements are a periodic function of their atomic number.’
Let us recall that the atomic number gives us the number of protons in the nucleus of an atom and this number increases by one in going from one element to the next. Elements, when arranged in order of increasing atomic number, lead us to the classification known as the Modern Periodic Table (Table ). Prediction of properties of elements could be made with more precision when elements were arranged on the basis of increasing atomic number.
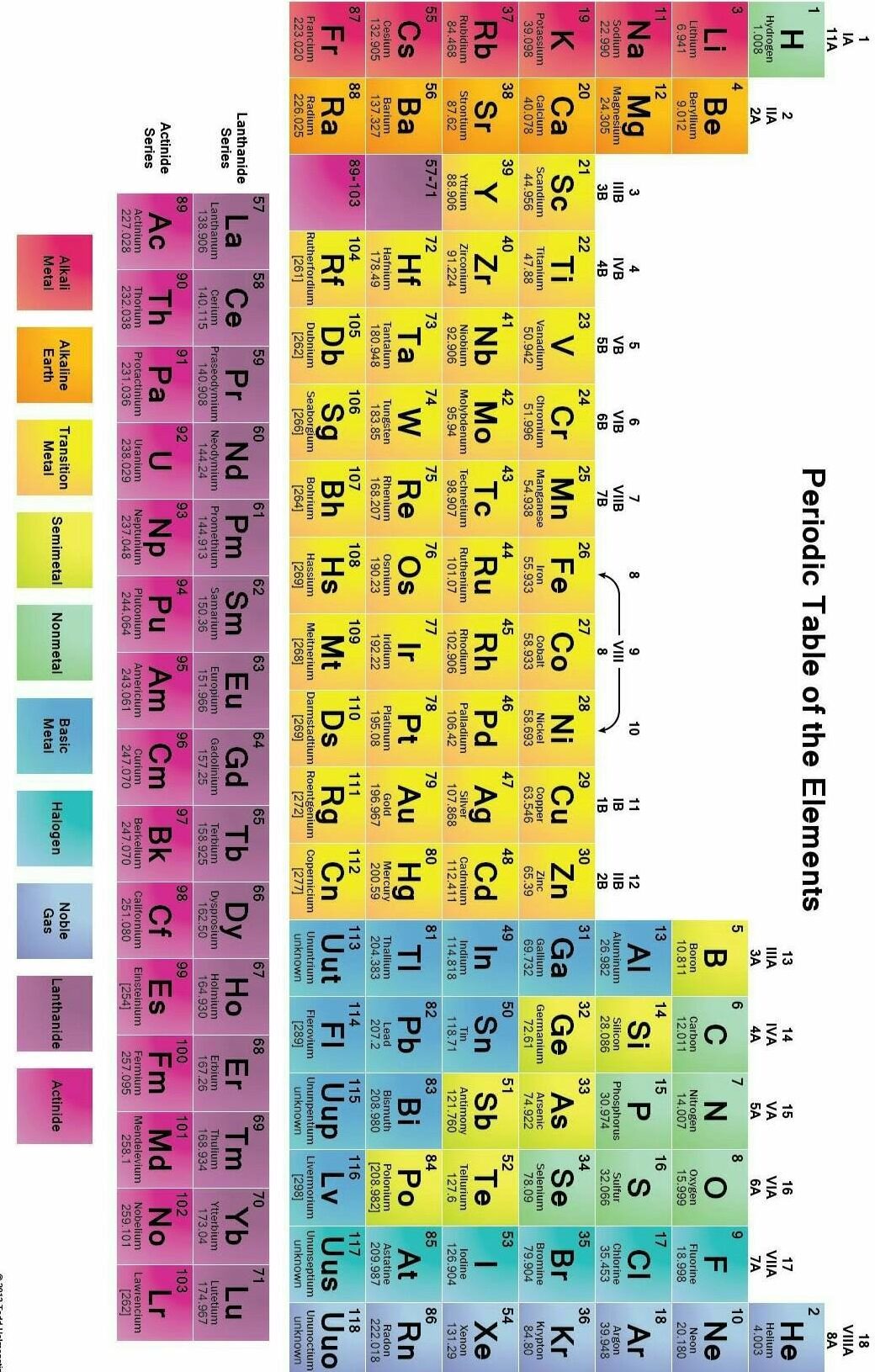
As we can see, the Modern Periodic Table takes care of three limitations of Mendléev’s Periodic Table. The anomalous position of hydrogen can be discussed after we see what are the bases on which the position of an element in the Modern Periodic Table depends.
Position of Elements in the Modern Periodic Table
The Modern Periodic Table has 18 vertical columns known as ‘groups’ and 7 horizontal rows known as ‘periods’. Let us see what decides the placing of an element in a certain group and period.
You will find that all these elements contain the same number of valence electrons. Similarly, you will find that the elements present in any one group have the same number of valence electrons. For example, elements fluorine (F) and chlorine (Cl), belong to group 17, how many electrons do fluorine and chlorine have in their outermost shells? Hence, we can say that groups in the Periodic Table signify an identical outer shell electronic configuration. On the other hand, the number of shells increases as we go down the group.
There is an anomaly when it comes to the position of hydrogen because it can be placed either in group 1 or group 17 in the first period.
You will find that these elements of second period do not have the same number of valence electrons, but they contain the same number of shells. You also observe that the number of valence shell electrons increases by one unit, as the atomic number increases by one unit on moving from left to right in a period.
Or we can say that atoms of different elements with the same number of occupied shells are placed in the same period. Na, Mg, Al, Si, P, S, Cl and Ar belong to the third period of the Modern Periodic Table, since the electrons in the atoms of these elements are filled in K, L and M shells. Write the electronic configuration of these elements and confirm the above statement. Each period marks a new electronic shell getting filled.
How many elements are there in the first, second, third and fourth periods?
We can explain the number of elements in these periods based on how electrons are filled into various shells. You will study the details of this in higher classes. Recall that the maximum number of electrons that can be accommodated in a shell depends on the formula 2n2
For example,
K Shell – 2 × (1)2 = 2, hence the first period has 2 elements.
L Shell – 2 × (2)2 = 8, hence the second period has 8 elements.
The third, fourth, fifth, sixth and seventh periods have 8, 18, 18, 32 and 32 elements respectively.
where ‘n’ is the number of the given shell from the nucleus.
The position of an element in the Periodic Table tells us about its chemical reactivity. As you have learnt, the valence electrons determine the kind and number of bonds formed by an element. Can you now say why Mendeléev’s choice of formulae of compounds as the basis for deciding the position of an element in his Table was a good one? How
would this lead to elements with similar chemical properties being placed in the same group?
Trends in the Modern Periodic Table
Valency : As you know, the valency of an element is determined by the number of valence electrons present in the outermost shell of its atom.
Atomic size: The term atomic size refers to the radius of an atom. The atomic size may be visualised as the distance between the centre of the nucleus and the outermost shell of an isolated atom. The atomic radius of hydrogen atom is 37 pm (picometre, 1 pm = 10–12m).
Let us study the variation of atomic size in a group and in a period.
You will see that the atomic radius decreases in moving from left to right along a period. This is due to an increase in nuclear charge which tends to pull the electrons closer to the nucleus and reduces the size of the atom.
You will see that the atomic size increases down the group. This is because new shells are being added as we go down the group. This increases the distance between the outermost electrons and the nucleus so that the atomic size increases in spite of the increase in nuclear charge.
Metallic and Non-metallic Properties
As we can see, the metals like Na and Mg are towards the left-hand side of the Periodic Table while the non-metals like sulphur and chlorine are found on the right-hand side. In the middle, we have silicon, which is classified as a semi-metal or metalloid because it exhibits some properties of both metals and non-metals.
In the Modern Periodic Table, a zig-zag line separates metals from non-metals. The borderline elements – boron, silicon, germanium, arsenic, antimony, tellurium and polonium – are intermediate in properties and are called metalloids or semi-metals.
Metals tend to lose electrons while forming bonds, that is, they are electropositive in nature.
As the effective nuclear charge acting on the valence shell electrons increases across a period, the tendency to lose electrons will decrease. Down the group, the effective nuclear charge experienced by valence electrons is decreasing because the outermost electrons are farther away from the nucleus. Therefore, these can be lost easily. Hence metallic character decreases across a period and increases down a group.
Non-metals, on the other hand, are electronegative. They tend to form bonds by gaining electrons. Let us learn about the variation of this property.
As the trends in the electronegativity show, non-metals are found on the right-hand side of the Periodic Table towards the top.
These trends also help us to predict the nature of oxides formed by the elements because it is known to you that the oxides of metals are basic and that of non-metals are acidic in general.
