Properties of Ionic Compounds
You may have observed the following general properties for ionic compounds—
(i) Physical nature: Ionic compounds are solids and are somewhat hard because of the strong force of attraction between the positive and negative ions. These compounds are generally brittle and break into pieces when pressure is applied.
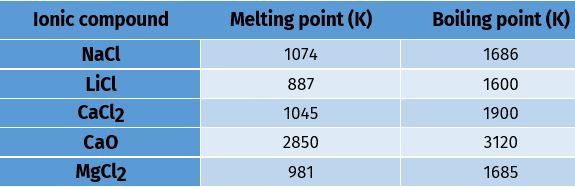
(ii) Melting and Boiling points: Ionic compounds have high melting and boiling points . This is because a considerable amount of energy is required to break the strong inter-ionic attraction.
(iii) Solubility: Electrovalent compounds are generally soluble in water and insoluble in solvents such as kerosene, petrol, etc.
(iv) Conduction of Electricity: The conduction of electricity through a solution involves the movement of charged particles. A solution of an ionic compound in water contains ions, which move to the opposite electrodes when electricity is passed through the solution. Ionic compounds in the solid state do not conduct electricity because movement of ions in the solid is not possible due to their rigid structure. But ionic compounds conduct electricity in the molten state. This is possible in the molten state since the elecrostatic forces of attraction between the oppositely charged ions are overcome due to the heat. Thus, the ions move freely and conduct electricity.

Testing the conductivity of a salt solution
