What is a Displacement Reaction ?
“ The one atom or a set of atoms is displaced by another atom in a molecule is called displacement Reaction .”
A + BC → AC + B
The above equation , when A is more reactive than B.
A and B have to be either:
where in C indicates an anion.
Single Displacement Reaction :
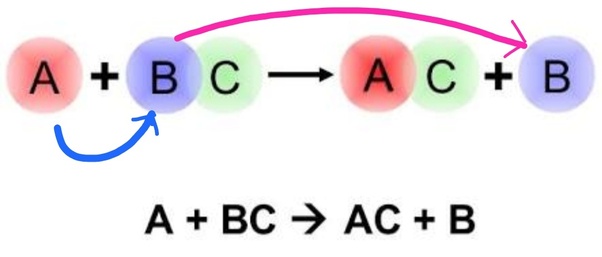
A single displacement reaction which is also called as single replacement reaction . when an ion or element moves out of a compound, i.e., one element is replaced by the other in a compound.
The following chemical reaction is example of Single Displacement Reaction –
Fe (s) + CuSO4 (aq) → FeSO4 (aq) + Cu(s)
(Copper sulphate) (Iron sulphate)
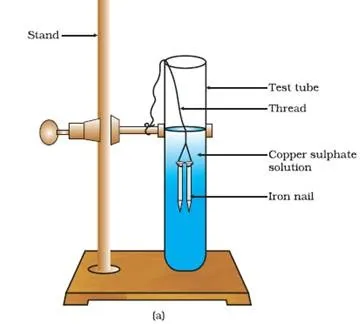
(a) Iron nails dipped in copper sulphate solution

In this reaction, iron has displaced or removed another element, copper, from copper sulphate solution. This reaction is known as displacement reaction.
Other examples of displacement reactions are
Zn(s + CuSO4 (aq) → ZnSO4 (aq) + Cu(s)
(Copper sulphate) (Zinc sulphate)
Pb(s) + CuCl2 (aq) → PbCl2 (aq) + Cu(s)
(Copper chloride) (Lead chloride)
Zinc and lead are more reactive elements than copper. They displace copper from its compounds.
Double Displacement Reaction:
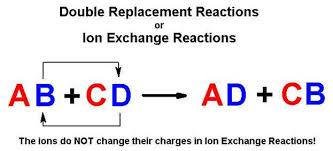
When a part of two ionic compounds is exchanged and makes two new components. The pattern of a double displacement of ions is called double displacement reaction .
In reaction , if product is formed insoluble in water. This insoluble substance formed is known as a precipitate. Any reaction that produces a precipitate can be called a precipitation reaction.
Na2SO4 (aq) + BaCl2 (aq) → BaSO4(s ) + 2NaCl (aq)
(Sodium (Barium (Barium (Sodium
sulphate) chloride) sulphate) chloride)
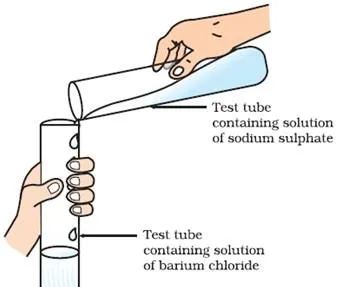
The white precipitate of BaSO4 is formed by the reaction of SO42- and Ba2+. The other product formed is sodium chloride which remains in the solution. Such reactions in which there is an exchange of ions between the reactants are called double displacement reactions.
