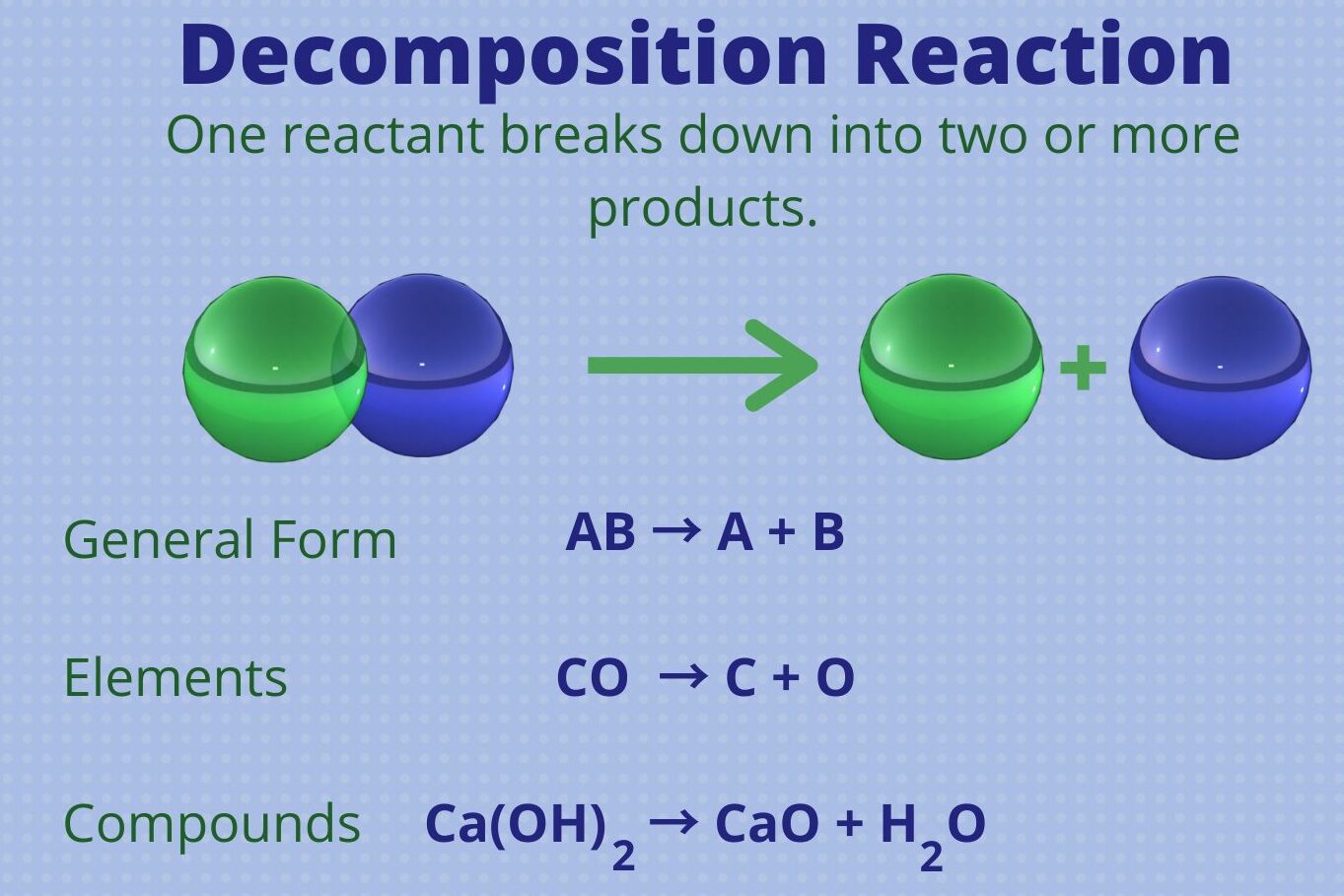
What is a Decomposition Reaction ?
Those chemical reaction in which one reactant breaks down into two or more products is called decomposition reaction.
General example of a decomposition reaction.
AB → A + B
Where A & B are the product molecules and AB is the reactant molecule.
Types of Decomposition reaction
Thermal decomposition
“When a decomposition reaction is carried out by heating, it is called thermal decomposition.“
The decomposition of calcium carbonate to calcium oxide and carbon dioxide on heating is an important decomposition reaction used in various industries. Calcium oxide is called lime or quick lime. It has many uses – one is in the manufacture of cement.
CaCO3 (s) + Heat → CaO (s) + CO2 (g)
(Limestone) (Quick lime)
e.g .
2FeSO4 (s) + Heat → Fe2O3 (s) + SO2 (g) + SO3 (g)
(Ferrous sulphate) (Ferric oxide)
In this reaction a single reactant breaks down to give simpler products. This is a decomposition reaction. Ferrous sulphate crystals (FeSO4 . 7H2O) lose water when heated and the colour of the crystals changes. It then decomposes to ferric oxide (Fe2O3), sulphur dioxide (SO2 ) and sulphur trioxide (SO3 ). Ferric oxide is a solid, while SO2 and SO3 are gases.
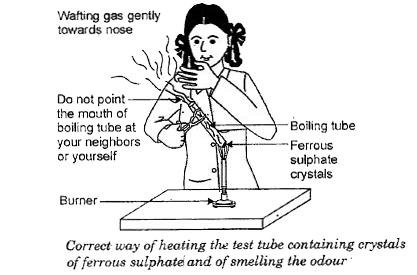
and of smelling the odour
eg
Heating of lead nitrate to form brown fumes. These fumes are of nitrogen dioxide (NO2 ). The reaction that takes place is –
2Pb(NO3)2 (s) + Heat → 2PbO(s) + 4NO2(g) + O2(g)
(Lead nitrate) (Lead oxide) (Nitrogen (Oxygen)
dioxide)
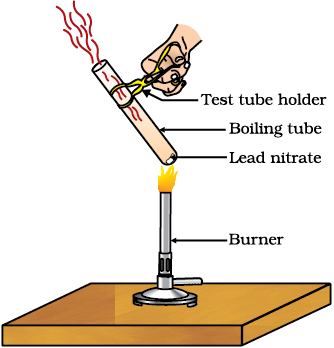
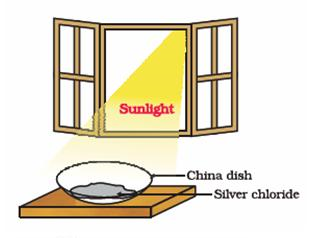
Photo decomposition / photolytic decomposition/ photochemical decomposition :
“When a decomposition reaction is carried out by light, it is called Photo decomposition.“
white silver chloride turns grey in sunlight. This is due to the decomposition of silver chloride into silver and chlorine by light.
2AgCl (s) + Sunlight → 2Ag (s) + Cl2 (g)
Silver bromide also behaves in the same way.
2AgBr(s) + Sunlight → 2Ag(s) + Br2 (g)
The above reactions are used in black and white photography.
Electrolytic decomposition reaction
An electrolytic decomposition reaction is a particular kind of decomposition reaction in which electrical energy serves as the activation energy for the decomposition process. The electrolysis of water is an illustration of an electrolytic decomposition reaction. Its chemical formula is 2H2O → 2H2 (g) + O2 (g) .

