MENDELÉEV’S PERIODIC TABLE
Dmitri Ivanovich Mendeléev, a Russian chemist. He was the most important contributor to the early development of a Periodic Table of elements wherein the elements were arranged on the basis of their fundamental property, the
atomic mass, and also on the similarity of chemical properties.
When Mendeléev started his work, 63 elements were known. He examined the relationship between the atomic masses of the elements and their physical and chemical properties. Among chemical properties, Mendeléev concentrated on the compounds formed by elements with oxygen and hydrogen. He selected hydrogen and oxygen as they are very reactive and formed compounds with most elements. The formulae of the hydrides and oxides formed by an element were treated as one of the basic properties of an element for its classification. He then took 63 cards and on each card he wrote down the properties of one element. He sorted out the elements with similar properties and pinned the cards together on a wall. He observed that most of the elements got a place in a Periodic Table and were arranged in the order of their increasing atomic masses. It was also observed that there occurs a periodic recurrence of elements with similar physical and chemical properties. On this basis, Mendeléev formulated a Periodic Law, which states that ‘the properties of elements are the periodic function of their atomic masses’.
Mendeléev’s Periodic Table contains vertical columns called ‘groups’ and horizontal rows called ‘periods’ (Table ).
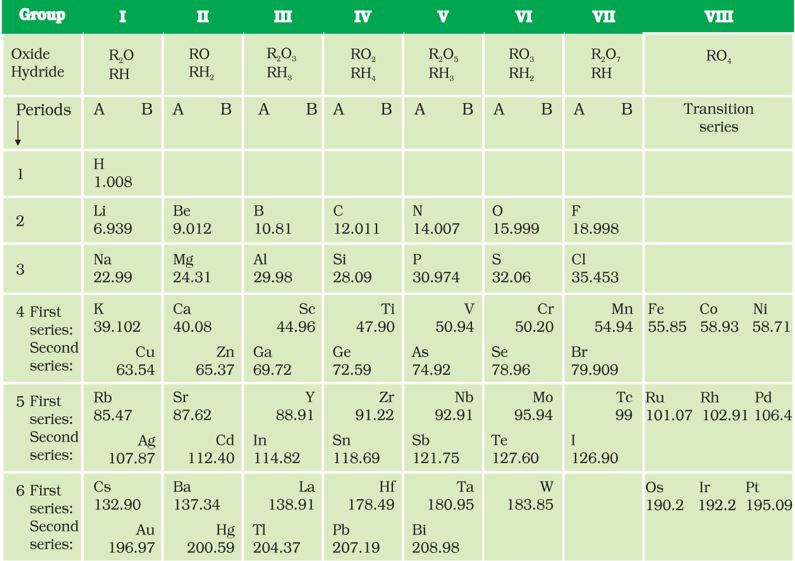
Mendeléev’s Periodic Table was published in a German journal in 1872. In the formula for oxides and hydrides at the top of the columns, the letter ‘R’ is used to represent any of the elements in the group. Note the way formulae are written. For example, the hydride of carbon, CH4 , is written as RH4
and the oxide CO2 , as RO2
.

Dmitri lvanovich Mendeléev (1834-1907)
Achievements of Mendeléev’s Periodic Table
While developing the Periodic Table, there were a few instances where Mendeléev had to place an element with a slightly greater atomic mass before an element with a slightly lower atomic mass. The sequence was inverted so that elements with similar properties could be grouped together. For example, cobalt (atomic mass 58.9) appeared before nickel (atomic mass 58.7). Looking at Table 5.4, can you find out one more such anomaly?
Further, Mendeléev left some gaps in his Periodic Table. Instead of looking upon these gaps as defects, Mendeléev boldly predicted the existence of some elements that had not been discovered at that time.
Mendeléev named them by prefixing a Sanskrit numeral, Eka (one) to the name of preceding element in the same group. For instance, scandium, gallium and germanium, discovered later, have properties similar to Eka–boron, Eka–aluminium and Eka–silicon, respectively. The properties of Eka–Aluminium predicted by Mendeléev and those of the
element, gallium which was discovered later and replaced Ekaaluminium, are listed as follows
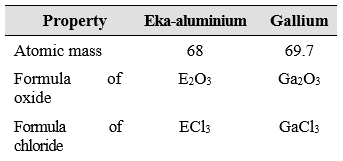
This provided convincing evidence for both the correctness and usefulness of Mendeléev’s Periodic Table. Further, it was the extraordinary success of Mendeléev’s prediction that led chemists not only to accept his Periodic Table but also recognise him, as the originator of the concept on which it is based. Noble gases like helium (He), neon (Ne) and argon (Ar) have been mentioned in many a context before this.
These gases were discovered very late because they are very inert and present in extremely low concentrations in our atmosphere. One of the strengths of Mendeléev’s Periodic Table was that, when these gases were discovered, they could be placed in a new group without disturbing the existing order.
Limitations of Mendeléev’s Classification
Electronic configuration of hydrogen resembles that of alkali metals. Like alkali metals, hydrogen combines with
halogens, oxygen and sulphur to form compounds having similar formulae, as shown in the examples here. On the other hand, just like halogens, hydrogen also exists as diatomic molecules and it combines with metals and non-metals to form covalent compounds.
Certainly, no fixed position can be given to hydrogen in the Periodic Table. This was the first limitation of Mendeléev’s Periodic Table. He could not assign a correct position to hydrogen in his Table.
Isotopes were discovered long after Mendeléev had proposed his periodic classification of elements. Let us recall that isotopes of an element have similar chemical properties, but different atomic masses.

Thus, isotopes of all elements posed a challenge to Mendeleev’s Periodic Law. Another problem was that the atomic masses do not increase in a regular manner in going from one element to the next. So it was not possible to predict how many elements could be discovered between two elements — especially when we consider the heavier elements.
