What is Oxidation?
Classical concept :
“The removal of hydrogen or any other electropositive element, or the addition of oxygen or , electronegative element, is said to be the process of oxidation . “
The process of loss of one or more electrons is known as oxidation, according to the electronic concept.
What is Reduction?
Classical concept :
“The removal of oxygen or any other electronegative element, or the addition of hydrogen or , electropositive element, is said to be the process of reduction . “
The process of gain of one or more electrons is known as reduction, according to the electronic concept.
Oxidising agent is a substance which brings about oxidation.
Reducing agent is a substance which brings about reduction.
The surface of copper powder becomes coated with black copper(II) oxide. Why has this black substance formed?
This is because oxygen is added to copper and copper oxide is formed.
2Cu + O2 + Heat → 2CuO
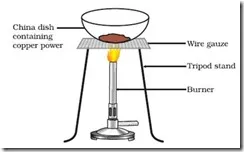
If hydrogen gas is passed over this heated material (CuO), the black coating on the surface turns brown as the reverse reaction takes place and copper is obtained.
CuO + H2+ Heat → Cu + H2O
If a substance gains oxygen during a reaction, it is said to be oxidised. If a substance loses oxygen during a reaction, it is said to be reduced.
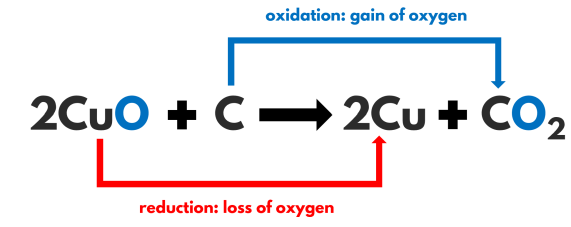
Oxidised substance – C
Reduced substance – CuO
Oxidising agent – CuO
Reducing agent – C
the copper(II) oxide is losing oxygen and is being reduced. The hydrogen is gaining oxygen and is being oxidised.
In other words, one reactant gets oxidised while the other gets reduced during a reaction. Such reactions are called oxidation-reduction reactions or redox reactions.
Some other examples of redox reactions are:
ZnO + C → + Zn + CO
MnO2 + HCl → MnCl2 + H 2O + Cl 2
