
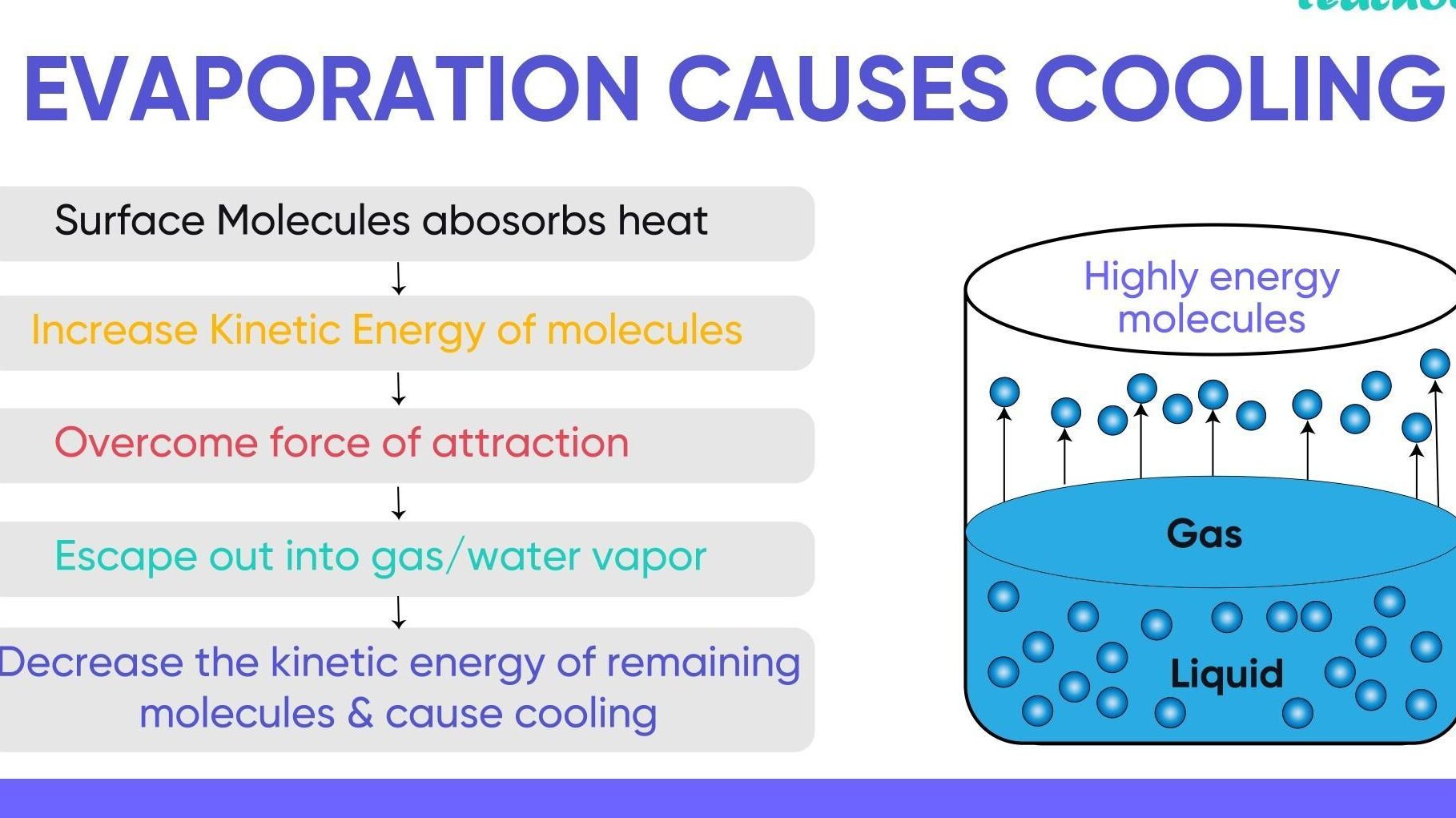
In an open vessel, the liquid keeps on evaporating. The particles of liquid absorb energy from the surrounding to regain the energy lost during evaporation. This absorption of energy from the surroundings make the surrounding.
when pour some acetone (nail polish remover) on palm, then feel cool.
The particles gain energy from your palm or surroundings and evaporate causing the palm to feel cool.
After a hot sunny day, people sprinkle water on the roof or open ground because the large latent heat of vaporisation of water
helps to cool the hot surface.
