Newlands’ Law of Octaves
The attempts of Döbereiner encouraged other chemists to correlate the properties of elements with their atomic masses. In 1866, John Newlands, n English scientist, arranged the then known elements in the order of increasing atomic masses. He started with the element having the lowest atomic mass (hydrogen) and ended at thorium which was the 56th element. He found that every eighth element had properties similar to that of the first. He compared this to the octaves found in music.
Therefore, he called it the ‘Law of Octaves’. It is known as ‘Newlands’ Law of Octaves’. In Newlands’ Octaves, the properties of lithium and sodium were found to be the same. Sodium is the eighth element after lithium. Similarly, beryllium and magnesium resemble each other. A part of the original form of Newlands’ Octaves is given in Table .
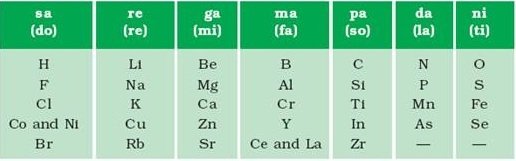
It was found that the Law of Octaves was applicable only up to calcium, as after calcium every eighth element did not possess properties similar to that of the first. n It was assumed by Newlands that only 56 elements existed in nature and no more elements would be discovered in the future. But, later on, several new elements were discovered, whose properties did not fit into the Law of Octaves. Newlands adjusted two elements in the same slot, but also put some unlike elements under the same note. Can you find examples of these from Table? Note that cobalt and nickel are in the same slot and these are placed in the same column as fluorine, chlorine and bromine which have very different properties than these elements. Iron, which resembles cobalt and nickel in properties, has been placed far away from these elements. With the discovery of noble gases, the Law of Octaves became irrelevant.
Thus, Newlands’ Law of Octaves worked well with lighter elements only.

Are you familiar with musical notes?
In the Indian system of music, there are seven musical notes in a scale – sa, re, ga, ma, pa, da, ni. In the west, they use the notations – do, re, mi, fa, so, la, ti. The notes in a scale are separated by whole and half-step frequency intervals of tones and semitones. A musician uses these notes for composing the music of a song. Naturally, there must be some repetition
of notes. Every eighth note is similar to the first one and it is the first note of the next scale.
Advantages and disadvantages of Law of Octave
Advantage-This law helped to arrange the elements with similar properties and provided a basis for classification.
Disadvantage-This law is only applicable up to Calcium as only 56 elements existed at the time he made this law.
