Metallic and Non-metallic Properties Trends in the Modern Periodic Table
The variation of metallic and nonmetallic properties along the period and along the group:
Moving from left to right across a period in the periodic table results in a decrease in metallic character. This happens because atoms are more willing to accept electrons in order to fill a valence shell than to lose them in order to empty the shell. As you move down a group, the metallic quality gets stronger.
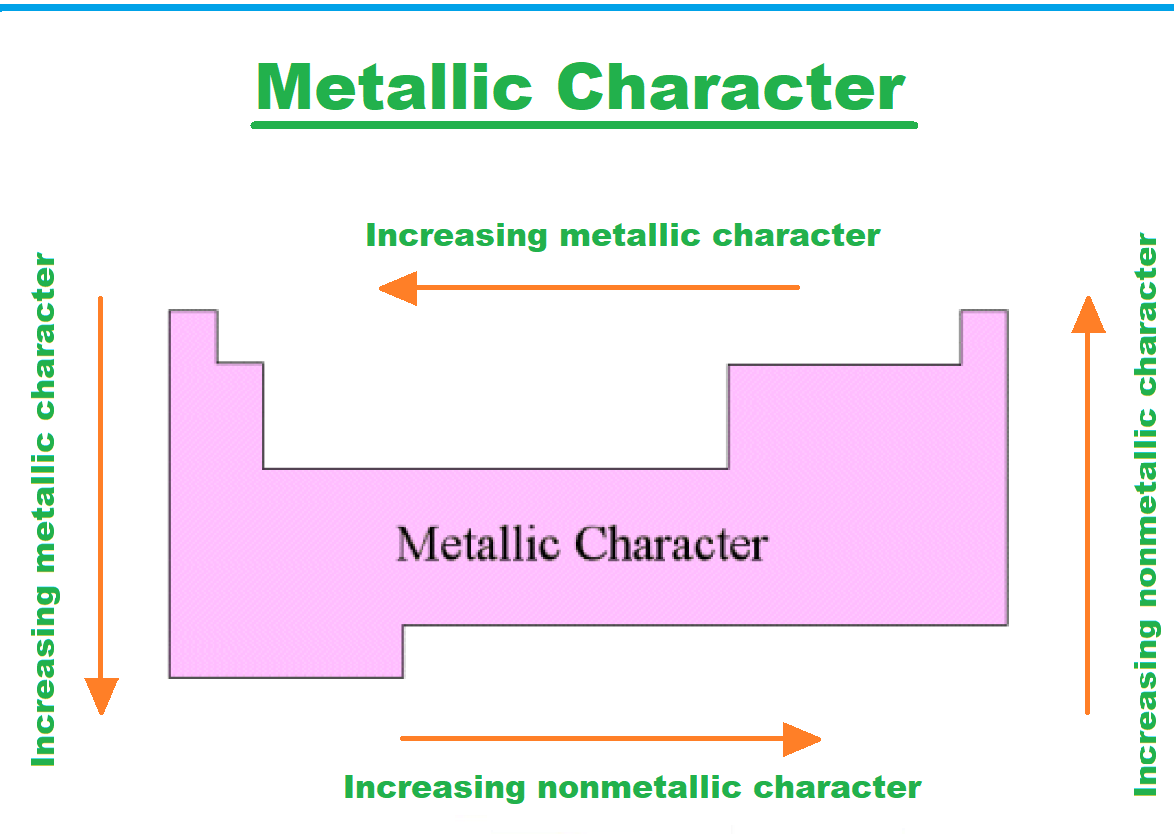
The ability of metals lower in a group to lose electrons increases as the ionisation energy decreases going down a group (or increases going up a group), making them more reactive. Additionally, as one descends a group, the atomic radius grows, pushing the outer electrons farther from the nucleus and decreasing their attraction to it. Metals thus become
