Döbereiner’s Triads
In the year 1817, Johann Wolfgang Döbereiner, a German chemist, tried to arrange the elements with similar properties into groups.
He identified some groups having three elements each. So he called these groups ‘triads’. Döbereiner showed that when the three elements in a triad were written in the order of increasing atomic masses; the atomic mass of the middle element was roughly the average of the atomic masses of the other two elements.
For example, take the triad consisting of lithium (Li), sodium (Na) and potassium (K) with the respective atomic masses of 6.9, 23.0 and 39.0. What is the average of the atomic masses of Li and K? How does this compare with the atomic mass of Na?
Given below Table are some groups of three elements. These elements are arranged downwards in order of increasing atomic masses.
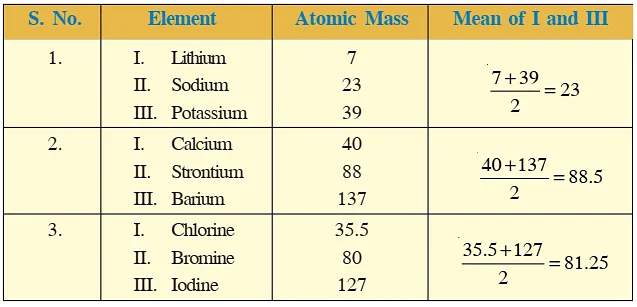
You will find that groups B and C form the Döbereiner triads. Döbereiner could identify only three triads from the elements known at that time (Table ). Hence, this system of classification into triads was not found to be useful.
Johann Wolfgang Döbereiner (1780-1849)
Johann Wolfgang Döbereiner studied as a pharmacist at Münchberg in Germany and then studied chemistry at Strasbourg. Eventually, he
became a professor of chemistry and pharmacy at the University of Jena. Döbereiner made the first observations on platinum as a catalyst and discovered similar triads of elements which led to
the development of the Periodic Table of elements.
The advantage is that he had thought to arrange the elements.
Disadvantages:
His classification was applicable only for a few elements.
He was not conformed to future predicted elements.
What he had given, all were his disadvantages So, there are no good advantages of his traits
