Let us consider two elements — calcium, atomic number 20, and argon, atomic number 18. The number of protons in these atoms is different, but the mass number of both these elements is 40. That is, the total number of nucleons is the same in the atoms of this pair of elements.
Atoms of different elements with different atomic numbers, which have the same mass number, are known as isobars.
Examples of isobars
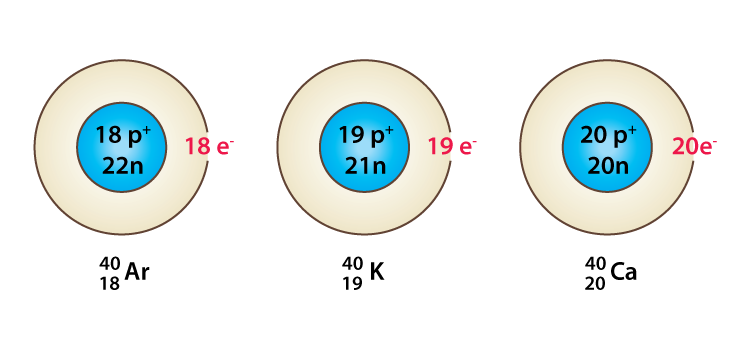
- Argon 18Ar40, potassium 19K40, and calcium 20Ca40 are examples of isobars.
- Here, 18 ,19 , and 20 are the atomic number of argon, potassium, and calcium respectively.
- All the above-mentioned atoms have the same mass number that is 40. However, their atomic numbers are different.
- Therefore, they are classified as isobars.
