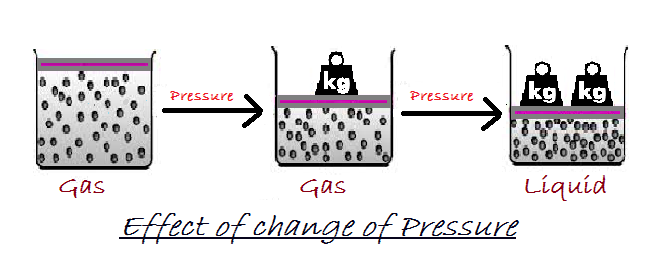
Temperature :
On increasing the temperature of solids, the kinetic energy of the particles increases.
Due to the increase in kinetic energy, the particles start vibrating with greater speed.
The energy supplied by heat overcomes the forces of attraction between the particles. The particles leave their fixed positions and start moving more freely. A stage is reached when the solid melts and is converted to a liquid.
The minimum temperature at which a solid melts to become a liquid at the atmospheric pressure is called its melting point. Heat gets used up in changing the state by overcoming the forces of attraction between the particles.
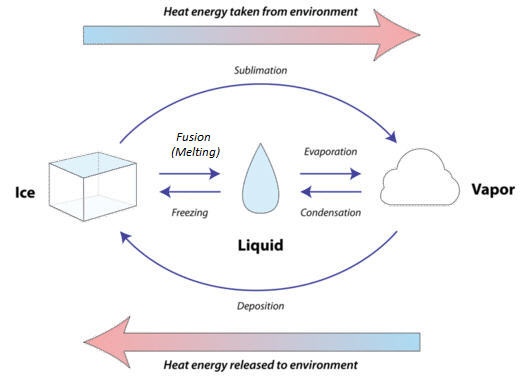
EFFECT OF CHANGE OF PRESSURE :
Various states of matter is due to the difference in the distances between the constituent particles. when we start putting pressure and compress a gas, Gas converted into liquids. But solid and liquid can not be compressed.