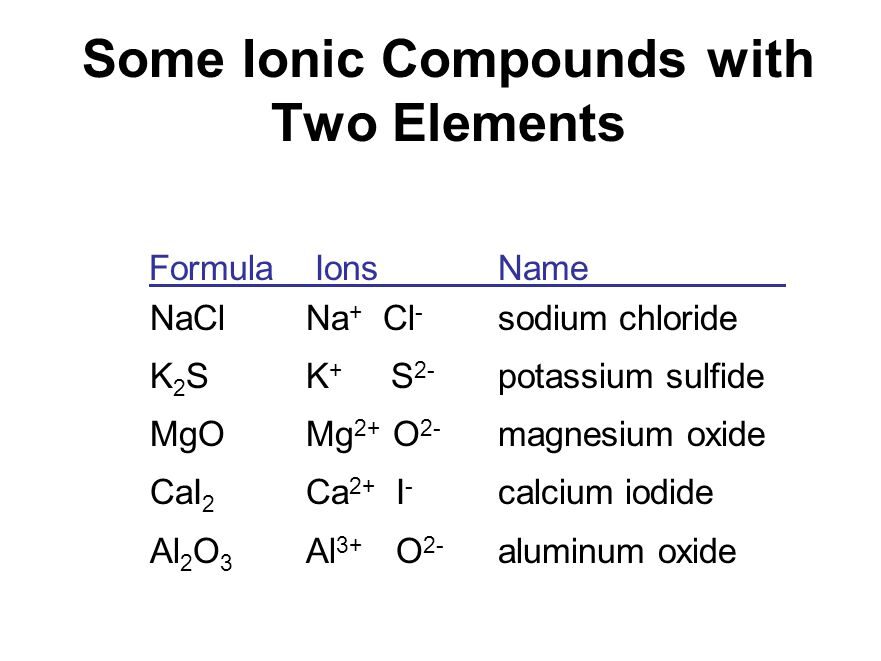
Compounds composed of metals and nonmetals contain charged species. The charged species are known as ions. Ions may consist of a single charged atom or a group of atoms that have a net charge on them. An ion can be negatively or positively charged. A negatively charged ion is called an ‘anion’ and the positively charged ion, a ‘cation’.
Take, for example, sodium chloride (NaCl). Its constituent particles are positively charged sodium ions (Na+ ) and negatively charged chloride ions (Cl–). A group of atoms carrying a charge is known as a polyatomic ion