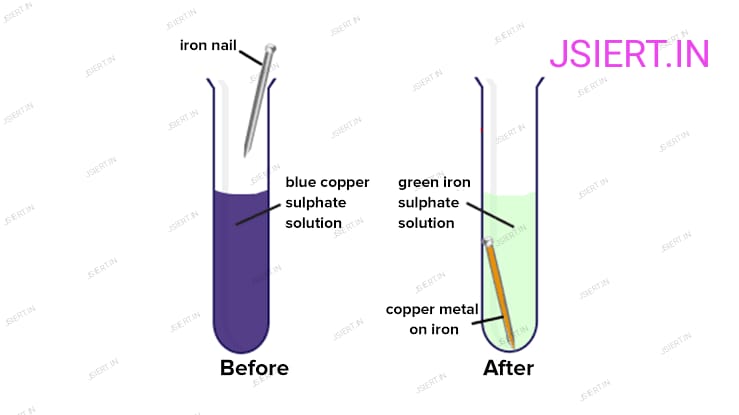
Because iron is more reactive than copper, it displaces copper from the copper sulphate solution when an iron nail is dipped in the solution. As a result, the copper sulphate solution’s colour changes. The result is
Fe + CuSO4 → FeSO4 + Cu