Diamond:
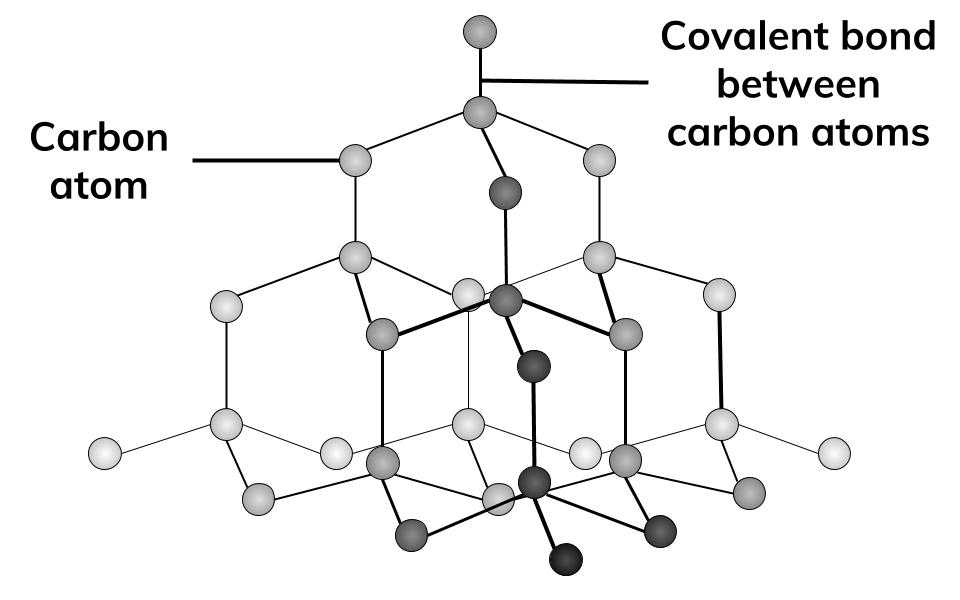
Each carbon atom in the crystalline lattice of a diamond is sp3 hybridised.
The length of the C-C covalent bond between each carbon atom and the other four carbon atoms is 154 pm.
A rigid 3-D network of carbon atoms with directional covalent bonds results from the structure’s extension into space.
Diamond is the earth’s hardest material because extended covalent bonding is very difficult to break.
Uses of diamonds
i) JEWELLERY
ii) INDUSTRIAL DIAMONDS
iii) AUTOMOTIVE INDUSTRY.
iv) Windows
v) Medicine
vi) Engraving
vii) Audio equipment
viii) Beauty products.
