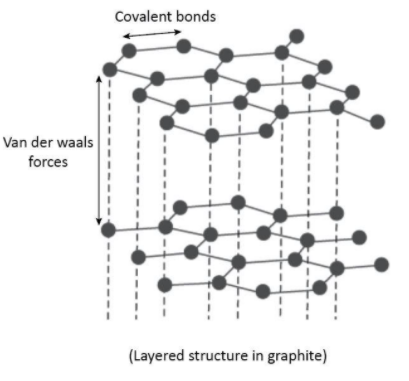
Graphite is soft and slippery, and its density is 2.3 g/cm3 . It is obvious that graphite is less dense than diamond, which makes it softer and more slippery.
Graphite is soft and slippery because the hexagonal structure of carbon atoms in graphite are bonded in layers with only weak Vanderwaal force holding the layers together. These layers are held together by weak Vander Waals forces, which make graphite soft and slippery.
