Solution
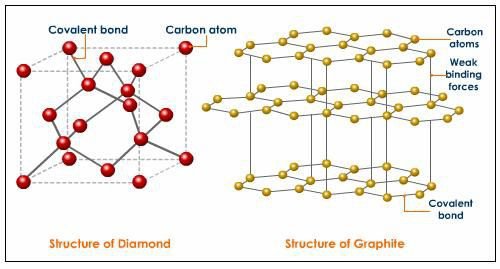
Diamond is hard because the carbon atoms in diamond are bonded in four covalent bonds in a stronger tetrahedron pattern, they form a giant crystalline structure, Hence diamond is hard,
but graphite is soft and slippery because the hexagonal structure of carbon atoms in graphite are bonded in layers with only weak vanderwaal force holding the layers together. These layers are held together by weak Vander Waals forces, which make graphite soft and slippery.
‘
