BONDING IN CARBON – THE COVALENT BOND
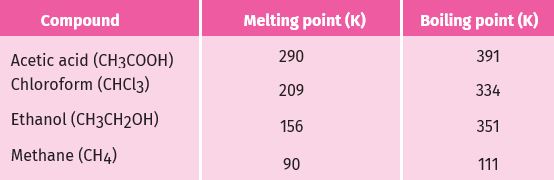
Most carbon compounds are poor conductors of electricity .The boiling and melting points of the carbon compounds, we find that these compounds have low melting and boiling points as compared to ionic compounds . We can conclude that the forces of attraction between the molecules are not very strong. Since these compounds are largely non-conductors of electricity, we can conclude that the bonding in these compounds does not give rise to any ions.
compounds of carbon
The combining capacity of various elements and how it depends on the number of valence electrons. Let us now look at the electronic configuration of carbon. The atomic number of carbon is 6. The reactivity of elements is explained as their tendency to attain a completely filled outer shell, that is, attain noble gas configuration. Elements forming ionic compounds achieve this by either gaining or losing electrons from the outermost shell. In the case of carbon, it has four electrons in its outermost shell and needs to gain or lose four electrons to attain noble gas configuration. If it were to gain or lose electrons –
(i) It could gain four electrons forming C4– anion. But it would be difficult for the nucleus with six protons to hold on to ten electrons, that is, four extra electrons.
(ii) It could lose four electrons forming C4+ cation. But it would require a large amount of energy to remove four electrons leaving behind a carbon cation with six protons in its nucleus holding on to just two electrons.
Carbon overcomes this problem by sharing its valence electrons with other atoms of carbon or with atoms of other elements. Not just carbon, but many other elements form molecules by sharing electrons in this manner. The shared electrons ‘belong’ to the outermost shells of both the atoms and lead to both atoms attaining the noble gas configuration.
Before going on to compounds of carbon, let us look at some simple molecules formed by the sharing of valence electrons.
The simplest molecule formed in this manner is that of hydrogen.
As you have learnt earlier, the atomic number of hydrogen is 1. Hence hydrogen has one electron in its K shell and it requires one more electron to fill the K shell. So two hydrogen atoms share their electrons to form a molecule of hydrogen, H2
. This allows each hydrogen atom to attain the electronic configuration of the nearest noble gas, helium, which has two electrons in its K shell. We can depict this using dots or crosses to represent valence electrons.
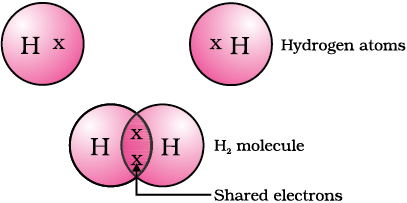
The shared pair of electrons is said to constitute a single covalent bond between the two hydrogen atoms.
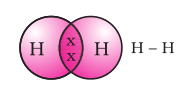
A single covalent bond is also represented by a line between the two atoms,
The atomic number of chlorine is 17. Chlorine forms a diatomic molecule, Cl2 .
.In the case of oxygen, we see the formation of a double bond between two oxygen atoms. This is because an atom of oxygen has six electrons in its L shell (the atomic number of oxygen is eight) and it requires two more electrons to complete its octet. So each atom of oxygen shares two electrons with another atom of oxygen to give us the structure .
The two electrons contributed by each oxygen atom give rise to two shared pairs of electrons. This is said to constitute a double bond between the two atoms.
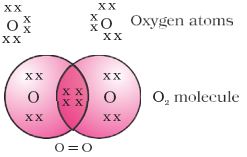
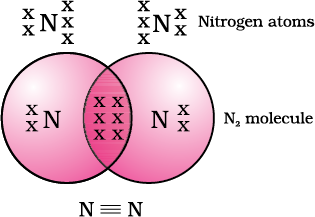
Nitrogen has the atomic number 7. What would be its electronic configuration and its combining capacity? In order to attain an octet, each nitrogen atom in a molecule of nitrogen contributes three electrons giving rise to three shared pairs of electrons. This is said to constitute a triple bond between the two atoms. The electron dot structure of N2 and its triple bond can be depicted .
A molecule of ammonia has the formula NH3. The molecule have single, double or triple bonds.
Let us now take a look at methane, which is a compound of carbon. Methane is widely used as a fuel and is a major
component of bio-gas and Compressed Natural Gas (CNG). It is also one of the simplest compounds formed by carbon. Methane has a formula CH4 . Hydrogen, as you know, has a valency of 1. Carbon is tetravalent because it has four valence electrons. In order to achieve noble gas configuration, carbon shares these electrons with four atoms of hydrogen as shown
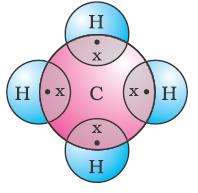
Such bonds which are formed by the sharing of an electron pair between two atoms are known as covalent bonds. Covalently bonded molecules are seen to have strong bonds within the molecule, but intermolecular forces are weak. This gives rise to the low melting and boiling points of these compounds. Since the electrons are shared between atoms and no charged particles are formed, such covalent compounds are generally poor conductors of electricity.
