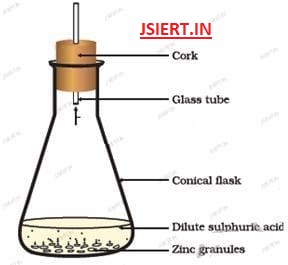
(a) Dilute sulphuric acid reacts with zinc granules to give zinc sulphate and hydrogen gas
Dilute sulphuric acid + zinc → Zinc Sulphate + Hydrogen Gas
H2SO4(aq) + Zn → ZnSO4(aq) + H2(g)
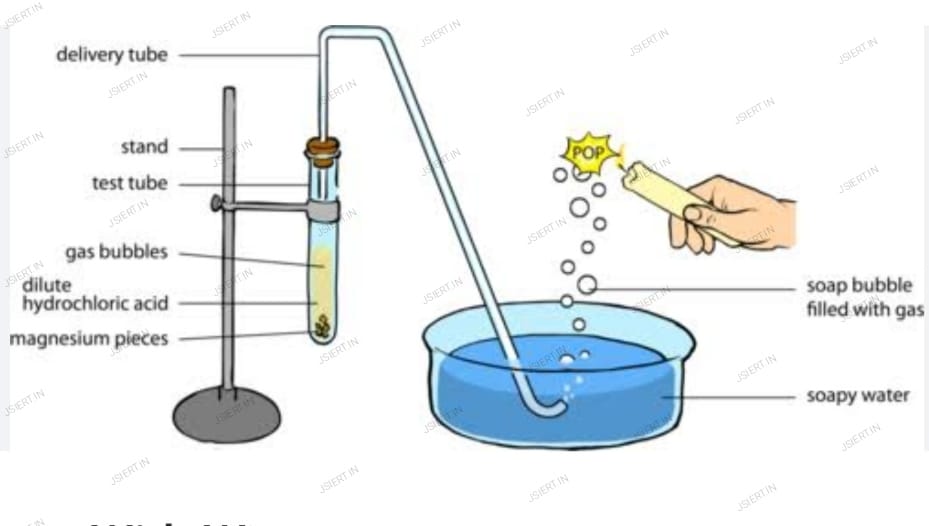
(b) Dilute hydrochloric acid reacts with magnesium ribbon to give magnesium oxide and hydrogen gas.
Dilute Hydrochloric + Magnesium → Magnesium Chloride + Hydrogen Gas
2HCl(aq) + Mg → MgCl2(aq) + H2(g)
(c) Dilute sulphuric acid reacts with aluminium powder to produce Aluminium Sulphate and Hydrogen Gas
Dilute Sulphuric Acid + Aluminium → Aluminium Sulphate + Hydrogen Gas
3H2SO4(aq) + 2Al(s) → Al2(SO4)3(aq) + 3H2(g)
(d) Dilute hydrochloric acid reacts with iron filings to produce Ferrous Chloride and Hydrogen Gas.
Dilute Hydrochloric Acid + Iron → Ferrous Chloride + Hydrogen Gas
6HCl(aq) + 3Fe(s) → 3FeCl2(aq) + 3H2(g)

