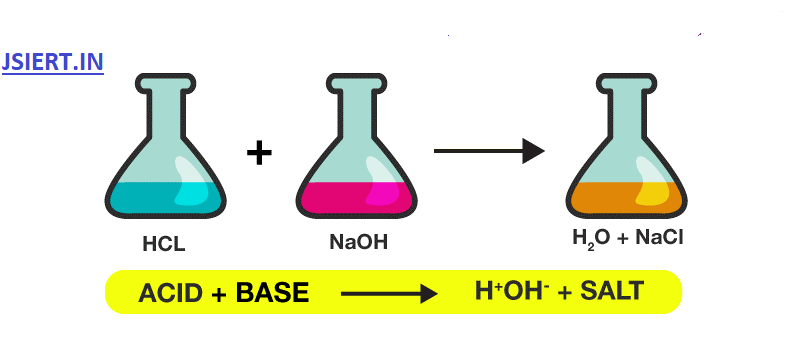
The reaction of the acid with base gives a product of corresponding salt and water, which is considered a neutralisation reaction.
e.g.
KOH + HCl → KCl + H2O
Ca(OH)2 + H2CO3 → CaCO3 + 2H2O

The reaction of the acid with base gives a product of corresponding salt and water, which is considered a neutralisation reaction.
e.g.
KOH + HCl → KCl + H2O
Ca(OH)2 + H2CO3 → CaCO3 + 2H2O