SOAPS AND DETERGENTS

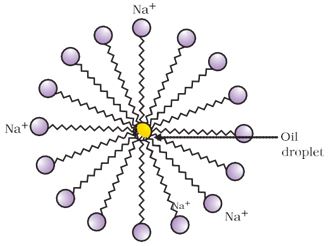

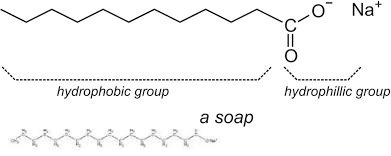
Soap : The effect of soap on cleaning. Most dirt is oily and as you know, oil does not dissolve in water. The molecules of soap are sodium or potassium salts of long-chain carboxylic acids. The ionic end of soap interacts with the water while the carbon chain interacts with oil. The soap molecules, thus form structures called micelles .where one end of the molecules is towards the oil droplet while the ionic end faces outside. This forms an emulsion in water. The soap micelle thus helps in pulling out the dirt in water and we can wash our clothes clean.
Detergent :
While bathing that foam is formed with difficulty and an insoluble substance (scum) remains after washing with water. This is caused by the reaction of soap with the calcium and magnesium salts, which cause the hardness of water. Hence you need to use a larger amount of soap. This problem is overcome by using another class of compounds called detergents as cleansing agents.
Detergents are generally sodium salts of sulphonic acids or ammonium salts with chlorides or bromides ions, etc. Both have long hydrocarbon chain. The charged ends of these compounds do not form insoluble precipitates with the calcium and magnesium ions in hard water. Thus, they remain effective in hard water. Detergents are usually used to make shampoos and products for cleaning clothes.