SOME IMPORTANT CARBON COMPOUNDS – ETHANOL AND ETHANOIC ACID
Many carbon compounds are invaluable to us. But here we shall study the properties of two commercially important compounds – ethanol and ethanoic acid.
Properties of Ethanol
Ethanol is a liquid at room temperature . Ethanol is commonly called alcohol and is the active ingredient of all alcoholic drinks. In addition, because it is a good solvent, it is also used in medicines such as tincture iodine, cough syrups, and many tonics. Ethanol is also soluble in water in all proportions. Consumption of small quantities of dilute ethanol causes drunkenness. Even though this practice is condemned, it is a socially widespread practice. However, intake of even a small quantity of pure ethanol (called absolute alcohol) can be lethal. Also, long-term consumption of alcohol leads to many health problems.
Reactions of Ethanol
(i) Reaction with sodium –
Alcohols react with sodium leading to the evolution of hydrogen. Give alkoxide and hydrogen gas . Ethanol reacts with sodium metal to produce sodium alkoxide and hydrogen gas.
2Na + 2CH3CH2OH → 2CH3CH2O–Na+ + H2
(Sodium ethoxide)

(ii) Reaction to give unsaturated hydrocarbon:
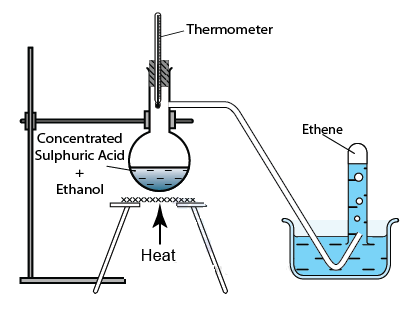
Heating ethanol at 443 K with excess concentrated sulphuric acid results in the dehydration of ethanol to give ethene –
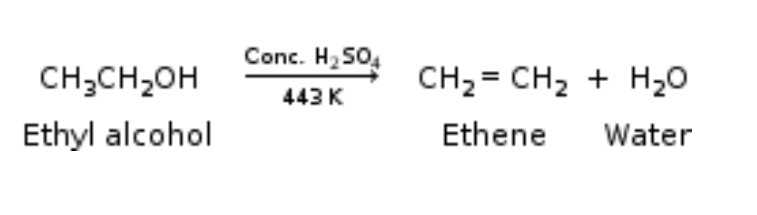
The concentrated sulphuric acid can be regarded as a dehydrating
agent which removes water from ethanol.
Properties of Ethanoic Acid
Ethanoic acid is commonly called acetic acid and belongs to a group of acids called carboxylic acids. 5-8% solution of acetic acid in water is called vinegar and is used widely as a preservative in pickles. The melting point of pure ethanoic acid is 290 K and hence it often freezes during winter in cold climates. This gave rise to its name glacial
acetic acid.
The group of organic compounds called carboxylic acids are obviously characterised by their acidic nature. However, unlike mineral acids like HCl, which are completely ionised, carboxylic acids are weak acids.
Reactions of ethanoic acid:
(i) Esterification reaction:
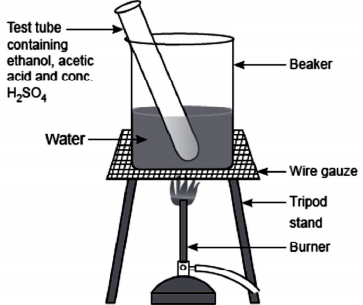
Esters are most commonly formed by reaction of an acid and an alcohol. Ethanoic acid reacts with absolute ethanol in the presence of an acid catalyst to give an ester –
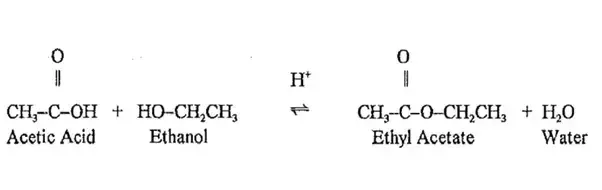
Generally, esters are sweet-smelling substances. These are used in making perfumes and as flavouring agents.
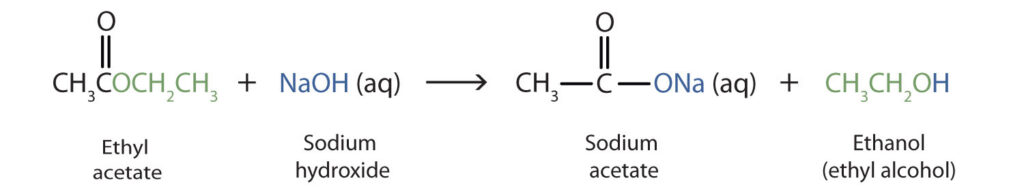
Saponification :

On treating with sodium hydroxide, which is an alkali, the ester is converted back to alcohol and sodium salt of carboxylic acid. This reaction is known as saponification because it is used in the preparation of soap. Soaps
are sodium or potassium salts of long chain carboxylic acid.
(ii) Reaction with a base:
Like mineral acids, ethanoic acid reacts with a base such as sodium hydroxide to give a salt (sodium ethanoate
or commonly called sodium acetate) and water:
NaOH + CH3COOH → CH3COONa + H2O
How does ethanoic acid react with carbonates and hydrogencarbonates?
(iii) Reaction with carbonates and hydrogencarbonates:
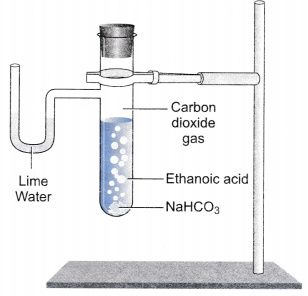
Ethanoic acid reacts with carbonates and hydrogencarbonates to give rise to a salt, carbon dioxide and water. The salt produced is commonly called sodium acetate.
2CH3COOH + Na2CO3 → 2CH3COONa + H2O + CO2
CH3COOH + NaHCO3 → CH3COONa + H2O + CO2
