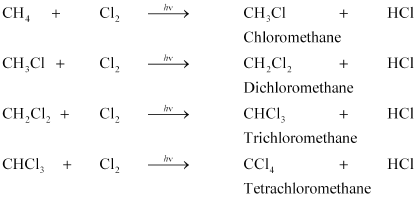
Substitution Reaction
Saturated hydrocarbons are fairly unreactive and are inert in the presence of most reagents. However, in the presence of sunlight, chlorine is added to hydrocarbons in a very fast reaction. Chlorine can replace the hydrogen
atoms one by one. It is called a substitution reaction because one type of atom or a group of atoms takes the place of another. A number of products are usually formed with the higher homologues of alkanes.
CH4 + Cl2 → CH3Cl + HCl (in the presence of sunlight)