Solution
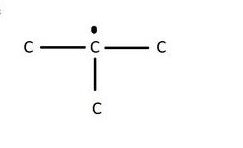
In graphite, out of the four valence electrons in a carbon atom, only three are used for covalent bonding with the other three carbon and the fourth is relatively free and can move from one carbon atom to the other. These free electrons make graphite a good conductor of electricity.
