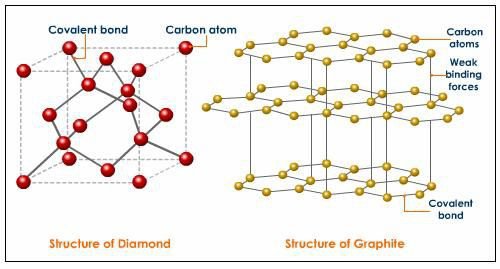
In Graphite
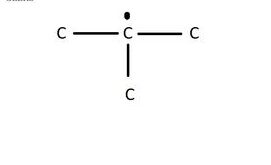
In a graphite molecule, one valence electron of each carbon atom remains free, due to the presence of a free electron; making graphite a good conductor of electricity.
In diamond
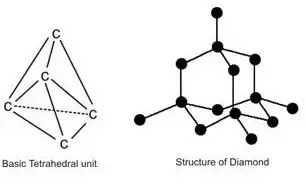
In diamond, they have no free mobile electron present in carbon atom. That is why diamond are bad conductor electricity.
