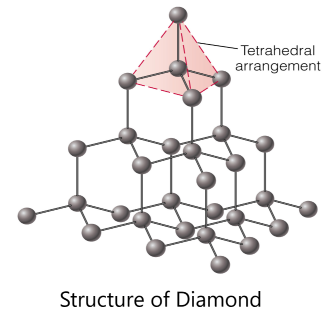
Solution –
Due to the tetrahedral arrangement of covalently bonded carbon atoms in diamonds, One carbon attaches to four carbon directly, there are no free electrons that can move and carry currents in diamonds. Since the conduction of electricity requires the presence of free electrons. Hence, diamond is a bad conductor of electricity.
Free electrons help in the conduction of electricity. In the 3-D structure of a diamond, each carbon atom is covalently bonded to four other carbon atoms thus, there are no free electrons and hence, it does not conduct electricity.
