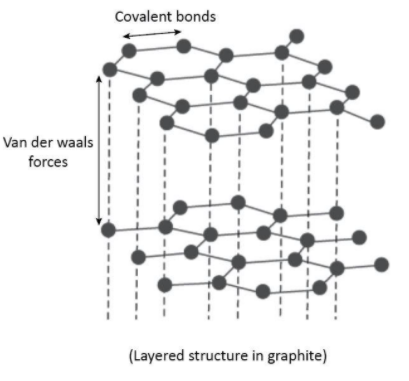
Graphite is a type of crystal carbon allotropes. It is a soft, black, flaky solid, a moderate electrical conductor. The carbon atoms are bonded in flat hexagonal lattices , which are then layered in sheets. In graphite, various hexagonal layers are held together by the weak van der Waal’s forces of attraction. The different graphite layers slip one over the other, it serves as a lubricant.