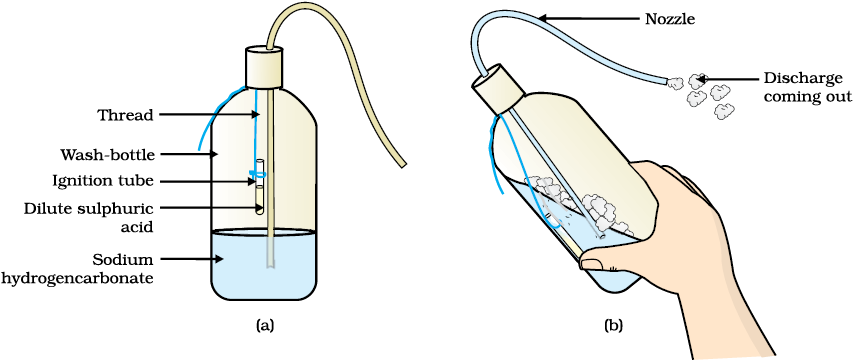
The reaction of acids with metal hydrogen carbonates is used in the fire extinguishers which produce carbon dioxide.
i) Take 20 ml of sodium hydrogen carbonate (NaHCO3) solution in a wash-bottle.
ii) Suspend an ignition tube containing dilute sulphuric acid in the wash-bottle.
iii) Close the mouth of the wash-bottle.
iv) Tilt the wash-bottle so that the acid from the ignition tube mixes with the sodium hydrogen carbonate solution below.
v) You will notice discharge coming out of the nozzle.
vi) Direct this discharge on a burning candle. What happens?
