
Plaster of Paris ( CaSO4 . 1/2 H2O )
On heating gypsum at 373 K, it loses water molecules and becomes calcium sulphate hemihydrate ( CaSO4 . 1/2 H2O ). This is called Plaster of Paris ( P.O.P),
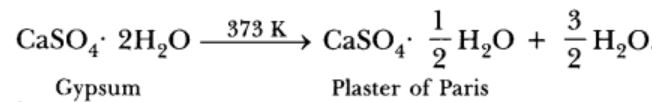
the substance which doctors use as plaster for supporting fractured bones in the right position. Plaster of Paris is a white powder and on mixing with water, it changes to gypsum once again giving a hard solid mass.
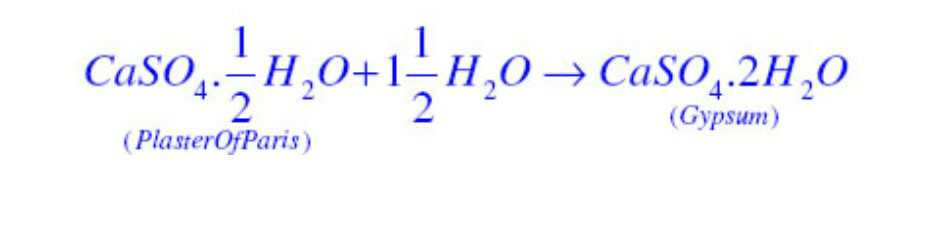
Note that only half a water molecule is shown to be attached as water of crystallisation. It is written in this form because two formula units of CaSO4 share one molecule of water.
Plaster of Paris is used for making toys, materials for decoration and for making surfaces smooth. Calcium sulphate hemihydrate is called ‘Plaster of Paris’
