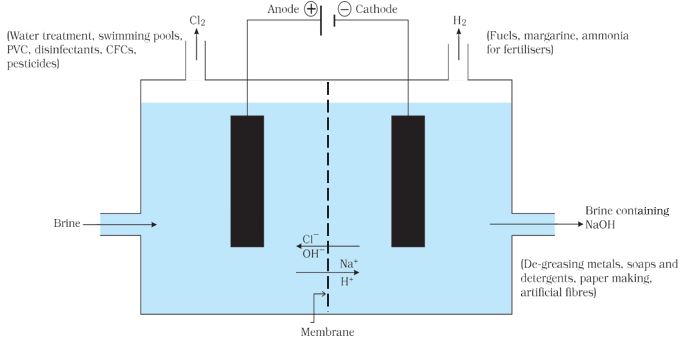
Sodium hydroxide ( NaOH )
When electricity is passed through an aqueous solution of sodium chloride (called brine), it decomposes to form sodium hydroxide. The process is called the chlor-alkali process because of the products formed– chlor for chlorine and alkali for sodium hydroxide.
2NaCl (aq) + 2H2O(l) → 2NaOH (aq) + Cl2 (g) + H2 (g)
Chlorine gas is given off at the anode, and hydrogen gas at the cathode. Sodium hydroxide solution is formed near the cathode. The three products produced in this process are all useful.