A scale for measuring hydrogen ion concentration in a solution, called pH scale has been developed. The p in pH stands for ‘potenz’ in German, meaning power.
pH Definition
Negative logarithm of the H+ or , H3O+ ion concentration.
pH = – log [H+]
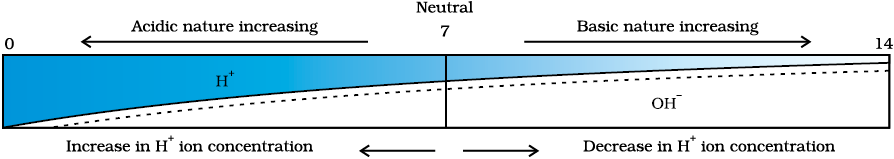
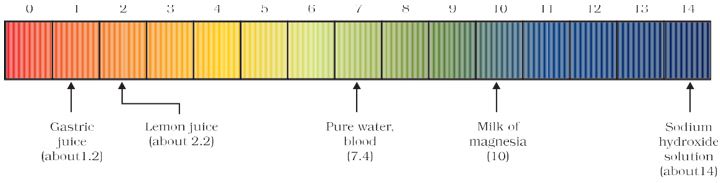
On the pH scale we can measure pH generally from 0 (very acidic) to 14 (very alkaline). pH should be thought of simply as a number which indicates the acidic or basic nature of a solution. Higher the hydronium ion concentration, lower is the pH value.
The pH of a neutral solution is 7. Values less than 7 on the pH scale represent an acidic solution. As the pH value increases from 7 to 14, it represents an increase in OH– ion concentration in the solution, that is, increase in the strength of alkali . Generally paper impregnated with the universal indicator is used for measuring pH.
The strength of acids and bases depends on the number of H+ ions and OH– ions produced, respectively. If we take hydrochloric acid and acetic acid of the same concentration, say one molar, then these produce different amounts of hydrogen ions. Acids that give rise to more H+ ions are said to be strong acids, and acids that give less H+ ions are said to be weak acids.
limitations of pH scale :
A solution’s scale pH value does not immediately indicate the relative strength of the solution.
i) For a 1N solution of a strong acid, pH is zero.
ii) Strong acid concentrations of 2N, 3N, and ION have a negative pH.
iii) At higher concentrations, Hammett acidity functions are used instead of pH.
