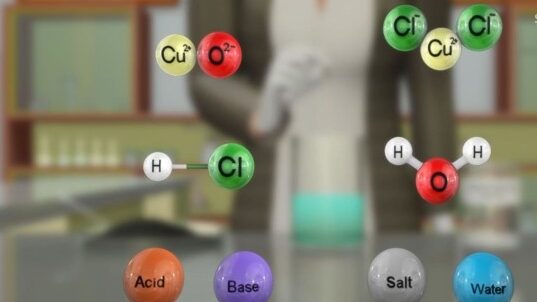
The colour of the solution becomes blue-green and the copper oxide dissolves. The blue-green colour of the solution is due to the formation of copper(II) chloride in the reaction.
Acids react with metal oxides to produce a salt and water.
The general reaction between a metal oxide and an acid can be written as –
Metal oxide + Acid → Salt + Water
For example:
2 HCl + CuO ⟶ CuCl 2 + H 2 O
Now write and balance the equation for the above reaction. Since metallic oxides react with acids to give salts and water, similar to the reaction of a base with an acid, metallic oxides are said to be basic oxides.
