when a magnesium ribbon is burnt in oxygen, it gets converted to magnesium oxide. This description of a chemical reaction in a sentence form is quite long. It can be written in a shorter form. The simplest way to do this is to write it in the form of a word-equation.
The word-equation for the above reaction would be –
Magnesium + Oxygen → Magnesium oxide
(Reactants) (Product)
The substances that undergo chemical change in the reaction, magnesium and oxygen, are the reactants. The new substance is magnesium oxide, formed during the reaction, as a product.
A word-equation shows change of reactants to products through an arrow placed between them. The reactants are written on the left-hand side (LHS) with a plus sign (+) between them. Similarly, products are written on the right-hand side (RHS) with a plus sign (+) between them.
The arrowhead points towards the products, and shows the direction of the reaction.
Writing a Chemical Equation

Chemical equations can be made more concise and useful if we use chemical formulae instead of words. A chemical equation represents a chemical reaction. If you recall formulae of magnesium, oxygen and
magnesium oxide, the above word-equation can be written as –
Mg + O2 → MgO
Count and compare the number of atoms of each element on the LHS and RHS of the arrow. Is the number of atoms of each element the same on both the sides? If yes, then the equation is balanced. If not, then the equation is unbalanced because the mass is not the same on both sides of the equation. Such a chemical equation is a skeletal
chemical equation for a reaction. Equation is a skeletal chemical equation for the burning of magnesium in air.
Balanced Chemical Equations
mass can neither be created nor destroyed in a chemical reaction. That is, the total mass of the elements present in the products of a chemical reaction has to be equal to the total mass of the elements present in the reactants.
In other words, the number of atoms of each element remains the same, before and after a chemical reaction. Hence, we need to balance a skeletal chemical equation.
Let us learn about balancing a chemical equation step by step.
The word-equation may be represented as –
Zinc + Sulphuric acid → Zinc sulphate + Hydrogen
The above word-equation may be represented by the following
chemical equation –
Zn + H2 SO4 → ZnSO4 + H2
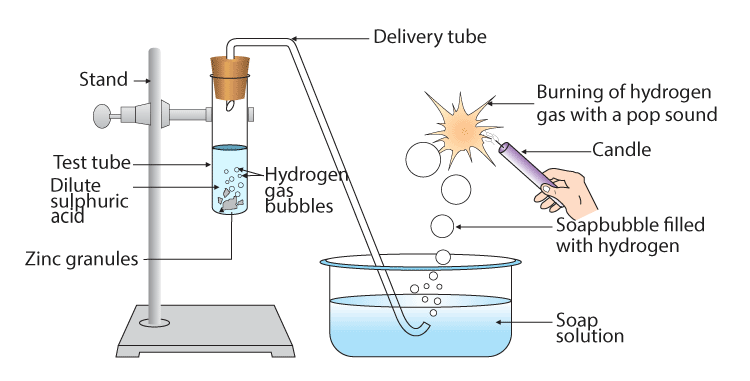
dilute sulphuric acid on zinc
Let us examine the number of atoms of different elements on both sides of the arrow.
Element
Zn
H
S
O
No. of atoms in LHS
1
2
1
4
No. of atoms in RHS
1
2
1
4
As the number of atoms of each element is the same on both sides of the arrow is a balanced chemical equation.
Let us try to balance the following chemical equation –
Fe + H2O → Fe3O4 + H2
Step I: To balance a chemical equation, first draw boxes around each formula. Do not change anything inside the boxes while balancing the equation.
Fe + H2O → Fe3O4 + H2
Step II: List the number of atoms of different elements present in the unbalanced equation .
Element
Fe
H
O
No. of atoms in LHS
1
2
1
No. of atoms RHS
3
2
4
Step III: It is often convenient to start balancing with the compound that contains the maximum number of atoms. It may be a reactant or a product. In that compound, select the element which has the maximum number of atoms. Using these criteria, we select Fe3O4 and the element oxygen in it. There are four oxygen atoms on the RHS and only one on the LHS.
Atoms of oxygen
(i) Initial
(ii)To balance
In reactants
1 (in H2O)
1 x 4
Products
4 (in Fe3O4)
4
To equalise the number of atoms, it must be remembered that we cannot alter the formulae of the compounds or elements involved in the reactions. For example, to balance oxygen atoms we can put coefficient ‘4’ as 4 H2O and not H2O4 or (H2O)4
. Now the partly balanced equation becomes –
Fe + 4 H2O → Fe3O4 + 4 H2
Step IV: Fe and H atoms are still not balanced. Pick any of these elements to proceed further. Let us balance hydrogen atoms in the partly balanced equation.
To equalise the number of H atoms, make the number of molecules of hydrogen as four on the RHS.
Atoms of hydrogen
(i) Initial
(ii) To balance
In reactant
8 (in 4 H2O)
8
In products
2 (in H2 )
2 x 4
The equation would be – Fe + 4 H2O → Fe3O4 + 4 H2 (partly balanced equation)
Step V: Examine the above equation and pick up the third element which is not balanced. You find that only one element is left to be balanced, that is, iron.
Atoms of Iron
(i) Initial
(ii) To balance
In reactants
1 (in Fe)
1 x 3
In products
3 (in Fe3O4 )
3
To equalise Fe, we take three atoms of Fe on the LHS.
3 Fe + 4 H2O → Fe3O4 + 4 H2
Step VI: Finally, to check the correctness of the balanced equation, we count atoms of each element on both sides of the equation.
3Fe + 4H2O → Fe3O4 + 4H2
The numbers of atoms of elements on both sides are equal. This equation is now balanced. This method of balancing chemical equations is called hit-and-trial method as we make trials to balance the equation by using the smallest whole number coefficient.
Step VII: Writing Symbols of Physical States . Does this equation tell us anything about the physical state of each reactant and product? No information has been given in this equation about their physical states. To make a chemical equation more informative, the physical states of the reactants and products are mentioned along with their chemical formulae. The gaseous, liquid, aqueous and solid states of reactants and products are represented by the notations (g), (l), (aq) and (s), respectively. The word aqueous (aq) is written if the reactant or product
is present as a solution in water.
The balanced Eq becomes
3Fe(s) + 4H2O(g) → Fe3O4 (s) + 4H2 (g)
Note that the symbol (g) is used with H2O to indicate that in this reaction water is used in the form of steam.
Usually physical states are not included in a chemical equation unless it is necessary to specify them.
Sometimes the reaction conditions, such as temperature, pressure, catalyst, etc., for the reaction are indicated above and/or below the arrow in the equation. For example
CO (g) + 2H2 (g) + 340atm → CH3 OH (l) ( Methyl alcohol )
6CO2 (aq) + 12H2 O (l) + Sunlight + Chlorophyll → C6H12O6 + 6O2
(Glucose) (Oxygen)
Using these steps, can you balance Eq.
