In nature, a number of atoms of some elements have been identified, which have the same atomic number but different mass numbers. For example, take the case of hydrogen atom, it has three atomic species, namely protium ( 1H1 ), deuterium ( 1 H 2 or D ) and tritium ( 1H3 or T ). The atomic number of each one is 1, but the mass number is 1, 2 and 3, respectively.
Other such examples are
(i) carbon, 6 C12 and 6 C12 , (ii) chlorine, 17 Cl 35 and 17 Cl 37 , etc.
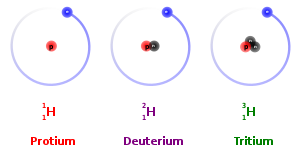
On the basis of these examples, isotopes are defined as the atoms of the same element, having the same atomic number but different mass numbers. Therefore, we can say that there are three isotopes of hydrogen atom, namely protium, deuterium and tritium.
Many elements consist of a mixture of isotopes. Each isotope of an element is a pure substance. The chemical properties of isotopes are similar but their physical properties are different.
Let us find out.
The average atomic mass of chlorine atom, on the basis of above data, will be
Average Atomic Mass. = (mass isotope A) x (% A) + ( mass isotope B) x (% B) …
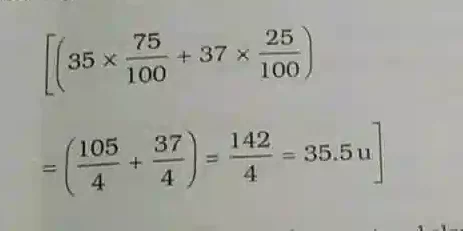
The mass of an atom of any natural element is taken as the average mass of all the naturally occuring atoms of that element. If an element
has no isotopes, then the mass of its atom would be the same as the sum of protons and neutrons in it. But if an element occurs in isotopic forms, then we have to know the percentage of each isotopic form and then the average mass is calculated.
This does not mean that any one atom of chlorine has a fractional mass of 35.5 u. It means that if you take a certain amount of chlorine, it will contain both isotopes of chlorine and the average mass is 35.5 u.
Applications of Isotopes
Since the chemical properties of all the isotopes of an element are the same, normally we are not concerned about taking a mixture. But some isotopes have special properties which find them useful in various fields. Some of them are :
(i) An isotope of uranium is used as a fuel in nuclear reactors.
(ii) An isotope of cobalt is used in the treatment of cancer.
(iii) An isotope of iodine is used in the treatment of goitre.
