Take an example of the reaction of hydrogen and oxygen to form water:
2H2 + O2 → 2H2O.
The above reaction indicates that
(i) two molecules of hydrogen combine with one molecule of oxygen to form two molecules of water, or
(ii) 4 u of hydrogen molecules combine with 32 u of oxygen molecules to form 36 u of water molecules.
We can infer from the above equation that the quantity of a substance can be characterised by its mass or the number of n molecules. But, a chemical reaction equation indicates directly the number of atoms or molecules taking part in the reaction.
Therefore, it is more convenient to refer to the quantity of a substance in terms of the number of its molecules or atoms, rather than their masses. So, a new unit “mole” was introduced.
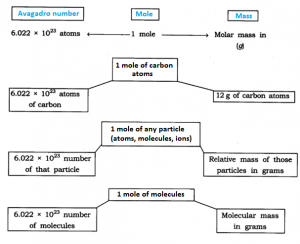
The mole, symbol mol, is the SI unit of amount of substance. One mole contains exactly 6.02214076 × 1023 elementary entities. This number is the fixed numerical value of the Avogadro constant, NA ,when expressed in the unit mol–1 and is called the Avogadro number*
The amount of substance, symbol n, of a system is a measure of the number of specified elementary entities. An elementary entity may
be an atom, a molecule, an ion, an electron, any other particle or specified group of particles. The mole is the amount of substance of a system that contains 6.02214076 × 1023 specified elementary entities.
1 mole (of anything) = 6.022 × 1023 in number,
as, 1 dozen = 12 nos.
1 gross = 144 nos
Besides being related to a number, a mole has one more advantage over a dozen or a gross. This advantage is that mass of 1 mole of a particular substance is also fixed.
The mass of 1 mole of a substance is equal to its relative atomic or molecular mass in grams. The atomic mass of an element gives us the mass of one atom of that element in atomic mass units (u). To get the mass of 1 mole of atom of that element, that is, molar mass, we have to take the same numerical value but change the units from ‘u’ to ‘g’. Molar mass of atoms is also known as gram atomic mass. For example, atomic mass of
hydrogen=1u. So, gram atomic mass of hydrogen = 1 g.
1 u hydrogen has only 1 atom of hydrogen 1 g hydrogen has 1 mole atoms, that is, 6.022 × 1023 atoms of hydrogen.
Similarly, 16 u oxygen has only 1 atom of oxygen, 16 g oxygen has 1 mole atoms, that is, 6.022 × 1023 atoms of oxygen.
To find the gram molecular mass or molar mass of a molecule, we keep the numerical value the same as the molecular mass, but simply change units as above from u to g. For example, as we have already calculated,
molecular mass of water (H2O) is 18 u. From
here we understand that
18 u water has only 1 molecule of water, 18 g water has 1 mole molecules of water, that is, 6.022 × 1023 molecules of water.
Chemists need the number of atoms and molecules while carrying out reactions, and for this they need to relate the mass in grams to the number. It is done as follows:
1 mole = 6.022 × 1023 number = Relative mass in grams.
Thus, a mole is the chemist’s counting unit.
The word “mole” was introduced around 1896 by Wilhelm Ostwald , who derived the term from the Latin word moles meaning a ‘heap’ or ‘pile’. A substance may be considered as a heap of atoms or molecules. The unit mole was accepted in 1967 to provide a simple way of reporting a large number– the massive heap of atoms and molecules in a sample.
.
